Hasan Atakan Bedel
M.Sc. Student in Department of Electrical and Electronics Engineering
Bilkent University
About
I am a Master’s student in the Electrical and Electronics Engineering department at Bilkent University, where I am fortunate to work with Prof. Tolga Cukur. I received my B.Sc. in Electrical and Electronics Engineering at Middle East Technical University.
My primary interest is in the field of biomedical data analysis, with a keen focus on harnessing the potential of deep learning techniques to revolutionize diagnostic and therapeutic methodologies. I am deeply committed to leveraging advanced AI algorithms to unlock novel insights from biomedical data, bridging the gap between cutting-edge technology and healthcare.
Latest version of my curriculum vitae is available here
Exciting Update: I am now actively seeking a Ph.D. program that aligns with my research interests!
- Deep Learning
- Medical Image Analysis
- Computational Neuroscience
M.Sc., Electrical and Electronics Engineering, 2021 - 2024
Bilkent University
B.Sc., Electrical and Electronics Engineering, 2016 - 2021
Middle East Technical University
Journal Publications
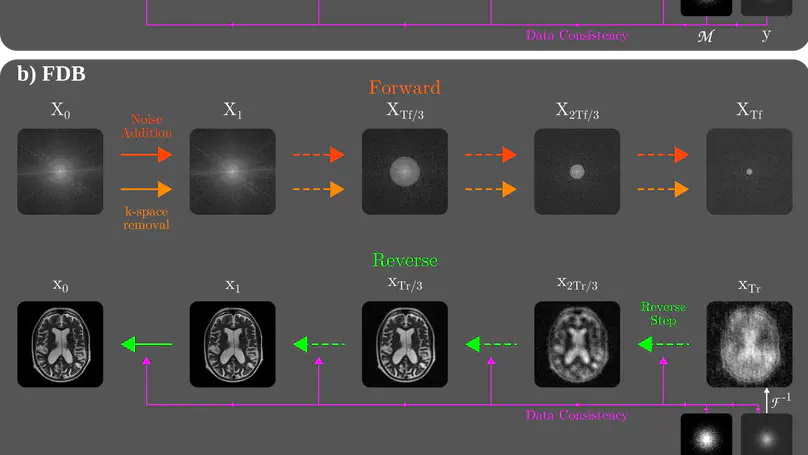
Recent years have witnessed a surge in deep generative models for accelerated MRI reconstruction. Diffusion priors in particular have gained traction with their superior representational fidelity and diversity. Instead of the target transformation from undersampled to fully-sampled data, common diffusion priors are trained to learn a multi-step transformation from Gaussian noise onto fully-sampled data. During inference, data-fidelity projections are injected in between reverse diffusion steps to reach a compromise solution within the span of both the diffusion prior and the imaging operator. Unfortunately, suboptimal solutions can arise as the normality assumption of the diffusion prior causes divergence between learned and target transformations. To address this limitation, here we introduce the first diffusion bridge for accelerated MRI reconstruction. The proposed Fourier-constrained diffusion bridge (FDB) leverages a generalized process to transform between undersampled and fully-sampled data via random noise addition and random frequency removal as degradation operators. Unlike common diffusion priors that use an asymptotic endpoint based on Gaussian noise, FDB captures a transformation between finite endpoints where the initial endpoint is based on moderate degradation of fully-sampled data. Demonstrations on brain MRI indicate that FDB outperforms state-of-the-art reconstruction methods including conventional diffusion priors.
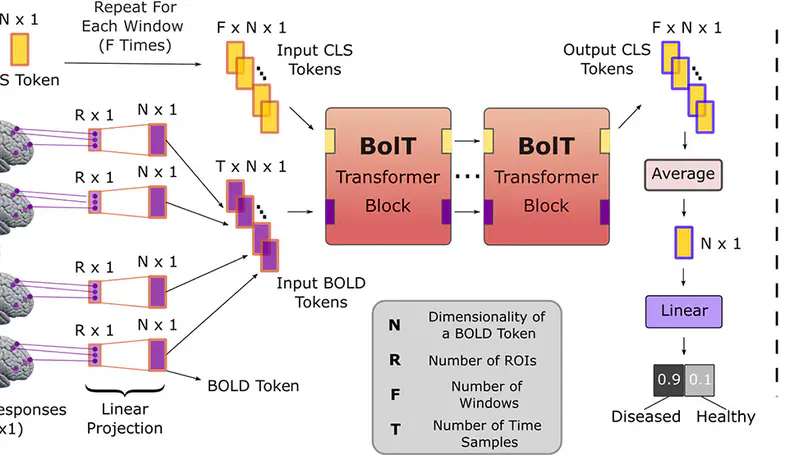
Deep-learning models have enabled performance leaps in analysis of high-dimensional functional MRI (fMRI) data. Yet, many previous methods are suboptimally sensitive for contextual representations across diverse time scales. Here, we present BolT, a blood-oxygen-level-dependent transformer model, for analyzing multi-variate fMRI time series. BolT leverages a cascade of transformer encoders equipped with a novel fused window attention mechanism. Encoding is performed on temporally-overlapped windows within the time series to capture local representations. To integrate information temporally, cross-window attention is computed between base tokens in each window and fringe tokens from neighboring windows. To gradually transition from local to global representations, the extent of window overlap and thereby number of fringe tokens are progressively increased across the cascade. Finally, a novel cross-window regularization is employed to align high-level classification features across the time series. Comprehensive experiments on large-scale public datasets demonstrate the superior performance of BolT against state-of-the-art methods. Furthermore, explanatory analyses to identify landmark time points and regions that contribute most significantly to model decisions corroborate prominent neuroscientific findings in the literature.
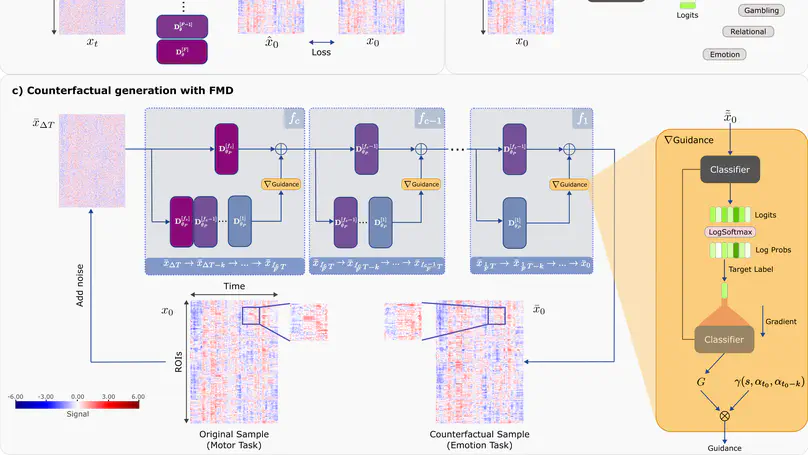
Deep learning analyses have offered sensitivity leaps in detection of cognitive states from functional MRI (fMRI) measurements across the brain. Yet, as deep models perform hierarchical nonlinear transformations on their input, interpreting the association between brain responses and cognitive states is challenging. Among common explanation approaches for deep fMRI classifiers, attribution methods show poor specificity and perturbation methods show limited plausibility. While counterfactual generation promises to address these limitations, previous methods use variational or adversarial priors that yield suboptimal sample fidelity. Here, we introduce the first diffusion-driven counterfactual method, DreaMR, to enable fMRI interpretation with high specificity, plausibility and fidelity. DreaMR performs diffusion-based resampling of an input fMRI sample to alter the decision of a downstream classifier, and then computes the minimal difference between the original and counterfactual samples for explanation. Unlike conventional diffusion methods, DreaMR leverages a novel fractional multi-phase-distilled diffusion prior to improve sampling efficiency without compromising fidelity, and it employs a transformer architecture to account for long-range spatiotemporal context in fMRI scans. Comprehensive experiments on neuroimaging datasets demonstrate the superior specificity, fidelity and efficiency of DreaMR in sample generation over state-of-the-art counterfactual methods for fMRI interpretation.
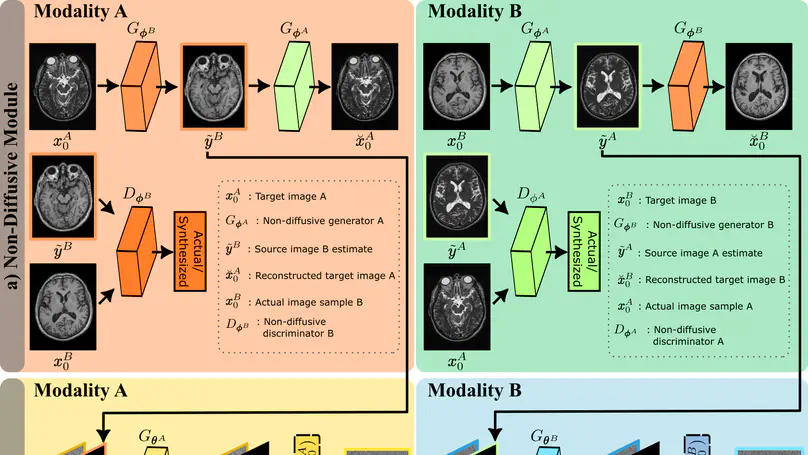
Imputation of missing images via source-to-target modality translation can improve diversity in medical imaging protocols. A pervasive approach for synthesizing target images involves one-shot mapping through generative adversarial networks (GAN). Yet, GAN models that implicitly characterize the image distribution can suffer from limited sample fidelity. Here, we propose a novel method based on adversarial diffusion modeling, SynDiff, for improved performance in medical image translation. To capture a direct correlate of the image distribution, SynDiff leverages a conditional diffusion process that progressively maps noise and source images onto the target image. For fast and accurate image sampling during inference, large diffusion steps are taken with adversarial projections in the reverse diffusion direction. To enable training on unpaired datasets, a cycle-consistent architecture is devised with coupled diffusive and non-diffusive modules that bilaterally translate between two modalities. Extensive assessments are reported on the utility of SynDiff against competing GAN and diffusion models in multi-contrast MRI and MRI-CT translation. Our demonstrations indicate that SynDiff offers quantitatively and qualitatively superior performance against competing baselines.
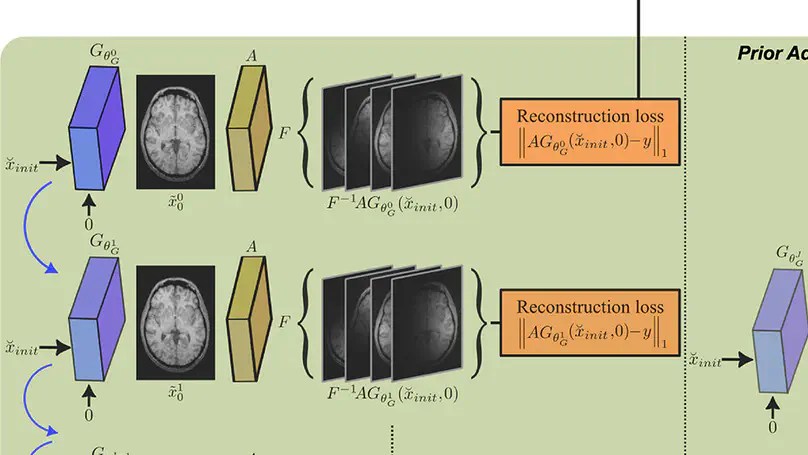
Deep MRI reconstruction is commonly performed with conditional models that de-alias undersampled acquisitions to recover images consistent with fully-sampled data. Since conditional models are trained with knowledge of the imaging operator, they can show poor generalization across variable operators. Unconditional models instead learn generative image priors decoupled from the operator to improve reliability against domain shifts related to the imaging operator. Recent diffusion models are particularly promising given their high sample fidelity. Nevertheless, inference with a static image prior can perform suboptimally. Here we propose the first adaptive diffusion prior for MRI reconstruction, AdaDiff, to improve performance and reliability against domain shifts. AdaDiff leverages an efficient diffusion prior trained via adversarial mapping over large reverse diffusion steps. A two-phase reconstruction is executed following training: a rapid-diffusion phase that produces an initial reconstruction with the trained prior, and an adaptation phase that further refines the result by updating the prior to minimize data-consistency loss. Demonstrations on multi-contrast brain MRI clearly indicate that AdaDiff outperforms competing conditional and unconditional methods under domain shifts, and achieves superior or on par within-domain performance.
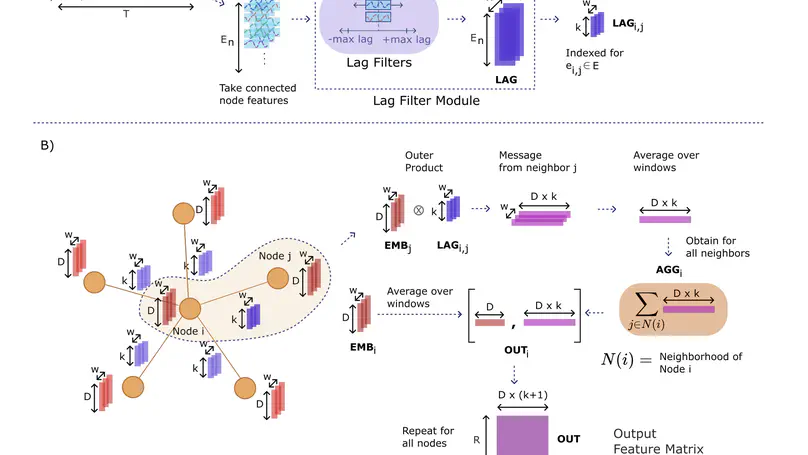
Deep learning analyses have offered sensitivity leaps in detection of cognitive states from functional MRI (fMRI) measurements across the brain. Yet, as deep models perform hierarchical nonlinear transformations on their input, interpreting the association between brain responses and cognitive states is challenging. Among common explanation approaches for deep fMRI classifiers, attribution methods show poor specificity and perturbation methods show limited plausibility. While counterfactual generation promises to address these limitations, previous methods use variational or adversarial priors that yield suboptimal sample fidelity. Here, we introduce the first diffusion-driven counterfactual method, DreaMR, to enable fMRI interpretation with high specificity, plausibility and fidelity. DreaMR performs diffusion-based resampling of an input fMRI sample to alter the decision of a downstream classifier, and then computes the minimal difference between the original and counterfactual samples for explanation. Unlike conventional diffusion methods, DreaMR leverages a novel fractional multi-phase-distilled diffusion prior to improve sampling efficiency without compromising fidelity, and it employs a transformer architecture to account for long-range spatiotemporal context in fMRI scans. Comprehensive experiments on neuroimaging datasets demonstrate the superior specificity, fidelity and efficiency of DreaMR in sample generation over state-of-the-art counterfactual methods for fMRI interpretation.





